
Cervical cancer can and should be prevented.
TruScreen provides an accurate, real-time screening solution. Our primary screening solution is ideal for communities that cannot access conventional, laboratory dependant, screening methods. It is affordable and easy to learn.
Our purpose is to ensure that all women of screening age, no matter who or where they are, have access to quality screening.
What is TruScreen?
The TruScreen device has been validated with large clinical studies including over 40,000 women in multiple settings.*
Browse published data

The smart investment

10 years
Established in 2013, TruScreen has been working towards its vision of A World Without Cervical Cancer.
Our People
1 billion +
Our market potential is huge with over a billion women eligible for screening in our key markets.
TruScreen's Markets
600,000+
WHO estimates that in 2020, there was 600,000+ new cases of cervical cancer worldwide.
Learn more about Cervical Cancer
40,000+
The TruScreen device has been clinically validated on over 40,000 women in multiple settings.*
MORE DATA
TruScreen Medical Advisory Committee Chairman
Associate Professor Michael J. Campion, TruScreen Medical Advisory Committee Chairman, sits down to discuss the science behind TruScreen’s Device, its clinical validation, and its application in real-world settings.
“My main clinical interest has been in the prevention of cervical cancer…it’s an amazing reality that perhaps the best of the biophysical screening tools, certainly for cervical cancer, has been developed entirely in Australia.”
Watch VideoCervical cancer is detectable and, if caught early, curable.
In low- and middle-income countries (LMICs) cervical cancer is the second most common form of cancer among women. About 90% of deaths from cervical cancer occur in LMICs.
The TruScreen device offers an alternative approach to cervical screening, resolving many of the ongoing issues with conventional screening methods, including accessibility and the need for supporting laboratory infrastructure.
Learn moreNews & Stories Highlights

News, 7.3.2023
Pitt Street Research Report
Market research company, Pitt Street, released its updated report on TruScreen.
View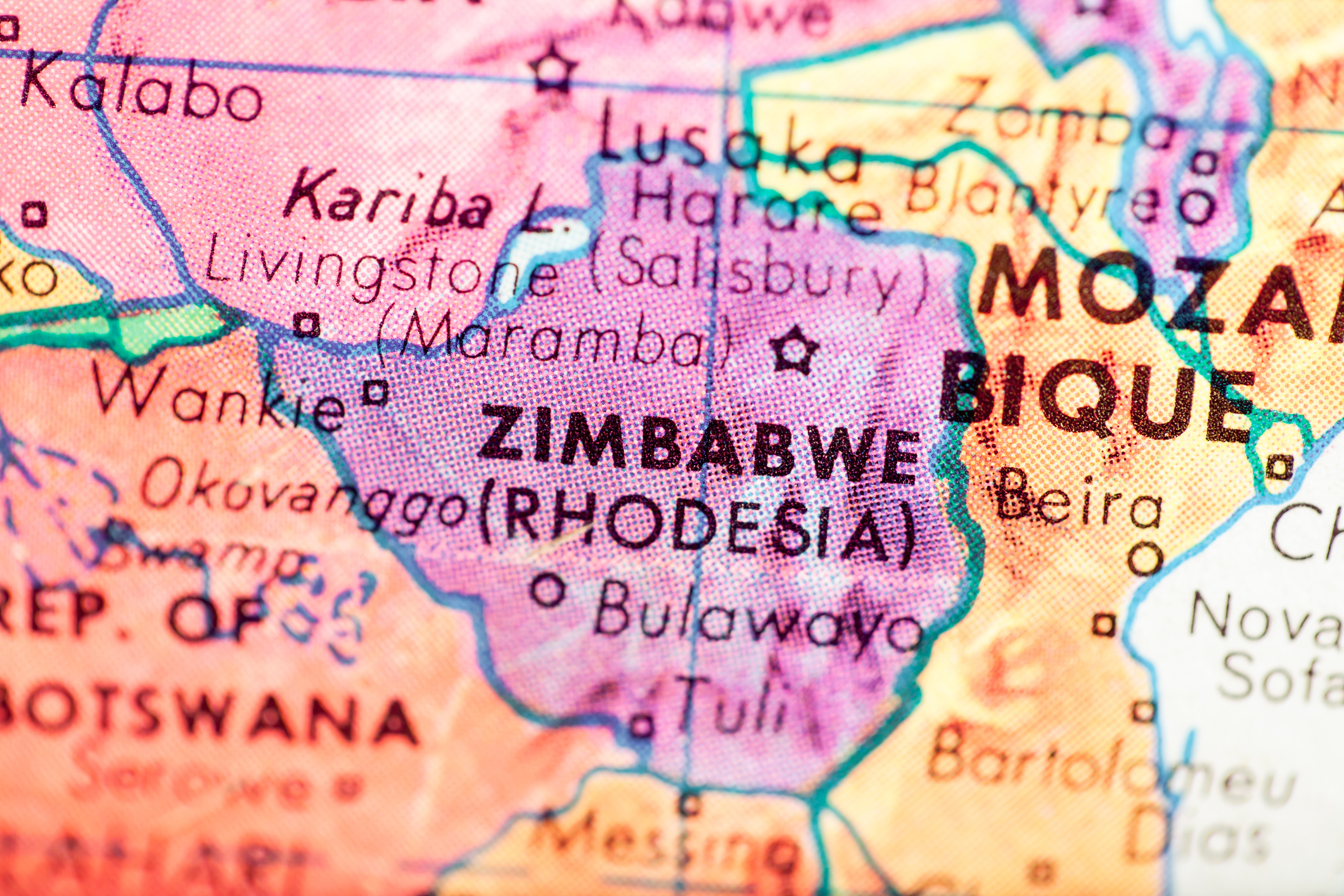
News, 9.2.2023
Zimbabwe second pilot project phase completed
The Zimbabwe National AIDS Council (NAC) wrapped up its current TruScreen pilot program in January. 10,000+ women were screened during the 6-month pilot. For many of these women this is
View
News, 27.1.2023
TruScreen outreach campaign in Mexico
At the end of 2022, TruScreen’s Mexican distributor in conjunction with a local foundation brought TruScreen screening to vulnerable young women.
View
News, 18.1.2023
TruScreen visits Vietnam
TruScreen’s Commercial GM travelled to Vietnam in late 2022 as boarders reopen and pandemic pressures begin to ease.
View
News, 15.12.2021
TruScreen continues to make progress in key markets worldwide
NZX/ASX Announcement 15 December 2021 TruScreen Group Limited (NZX/ASX: TRU) (‘TruScreen’ or ‘the Company’) is pleased to provide a market update, outlining the Company’s progress in several of its key
View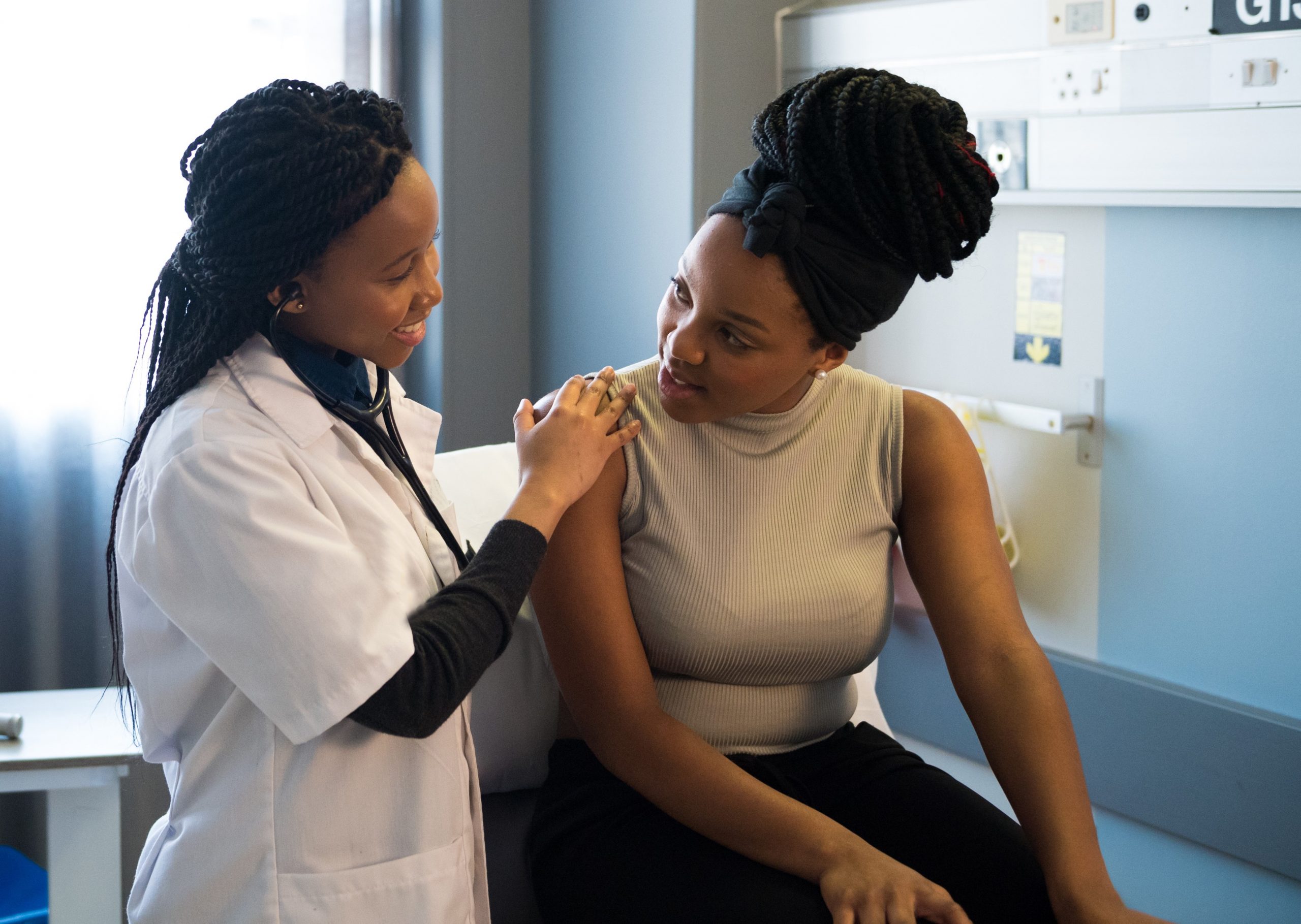
News, 22.10.2019
World Health Organisation sets global agenda to eliminate cervical cancer
Cervical cancer is one of the most preventable and treatable forms of cancer, yet over a quarter of a million women die from the disease each year. Ninety percent of
View
News, 10.12.2020
Zimbabwe’s dual public health crisis: cervical cancer and HIV
Zimbabwe may be known for its pristine wilderness, but it is also a country facing a major public health crisis in the form of high HIV and cervical cancer rates.
View
News, 16.7.2018
TruScreen’s New Production Facility Fully Operational & Delivers Significant Improvement In Device Profitability
Cervical cancer technology company, TruScreen Limited (NZAX:TRU), announces that its newly commissioned facility to manufacture the diagnostic Opto-electric front-end component of its device in Australia, is now fully operational.
View
News, 5.8.2019
TruScreen Training for Vietnam Ministry of Health Pilot Study Completed
Dr. Carolina Velasquez traveled to Vietnam to conduct clinical training and launch the TruScreen Pilot Study, being conducted out of Hanoi Gynaecology and Obstetrics Hospital. The Pilot Study, being overseen
View
News, 27.4.2020
TruScreen Market Acceptance – Russia
Our Russian distributor IntelMed Systems takes us into local hospitals where TruScreen pilot programmes are underway.
View
News, 9.1.2020
Social stigma restrains cervical cancer screening in China
Despite its rapidly growing economy, China remains a low- and middle-income nation. An example of this is its healthcare system. Although China has made impressive strides in improving healthcare access
View
News, 1.9.2021
TruScreen August ’21 Newsletter
Truscreen Group Limited (NZX/ASX: TRU) is pleased to provide a copy of its August 2021 Stakeholders Newsletter. This gives an update on TruScreen's performance and highlights key initiatives underway. Highlights
View
News, 12.6.2019
Cervical Cancer Technology Landscape – Unitaid & WHO Publication
As announced last month, TruScreen's cervical cancer screening device was recognised in a joint publication that Unitaid has released with the World Health Organisation and the Clinton Health Access Initiative.
View
News, 2.7.2021
First Sales Made in Eastern Europe
TruScreen Group Limited is pleased to advise that it has received its first order from Serbia, Eastern Europe.
View
News, 3.10.2019
Cervical Cancer Remains a Global Health Issue
Cervical cancer is the fourth most common cancer in women, with more than half a million women diagnosed with the disease in 2018. In low- and middle-income countries (LMIC’s) cervical
View
News, 26.10.2021
Wave of Health – the all-Russian Promotion
In September 2021, TruScreen’s Russian distributor, Intelmed Systems, participated in the 15th annual “Wave of Health” initiative. The annual “Wave of Health” campaign sees a ship with teams of doctors
View
News, 7.11.2019
Five Facts You May Not Know About Cervical Cancer
Cervical cancer affects millions of women worldwide. It is a silent killer, it has a high prevalence outside of developed countries, and almost all cases of cervical cancer can be
View
News, 27.11.2019
How TruScreen’s real-time cervical cancer screening works
TruScreen’s breakthrough cervical cancer screening device is a viable and attractive alternative to traditional cervical screening methods. The TruScreen device resolves many of the ongoing issues associated with Pap tests,
View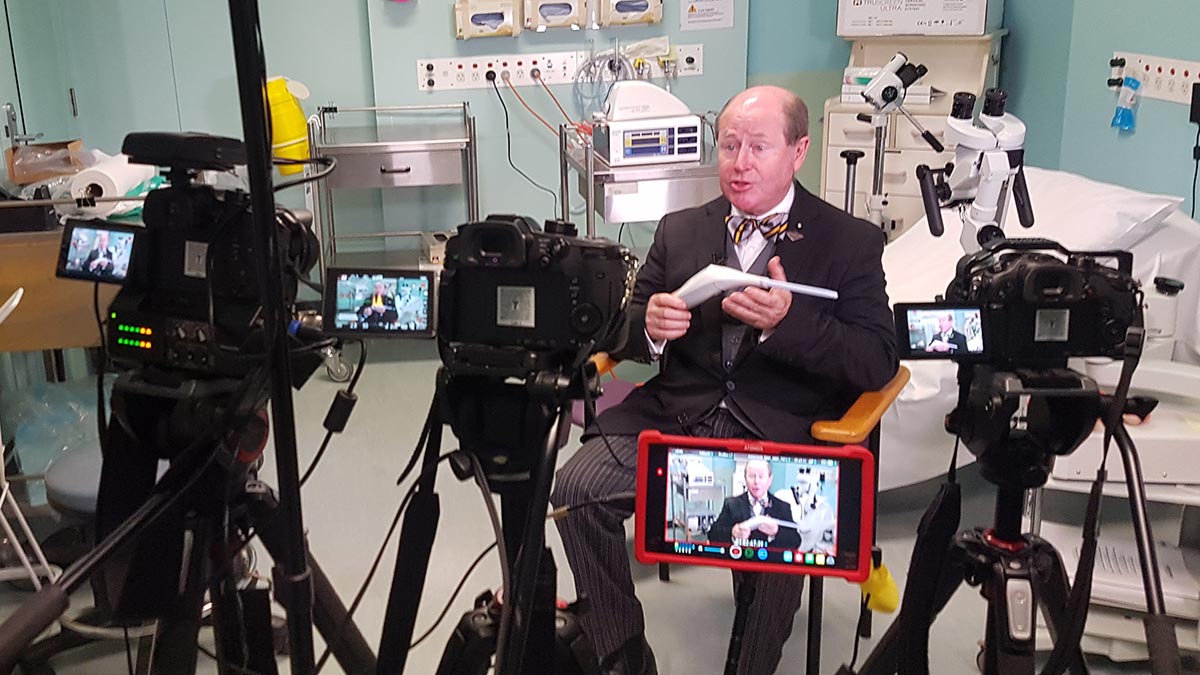
News, 15.5.2020
Video: TruScreen Clinical Validation and Application – Assoc. Prof. Michael J. Campion
Associate Professor Michael J. Campion, TruScreen Medical Advisory Committee member, sits down to discuss the science behind TruScreen’s Cervical Cancer Screening Device, its clinical validation, and its application in real-world
View
News, 6.9.2021
Zimbabwe Pilot Program Underway
The National Aids Council pilot will compare TruScreen to VIAC. VIAC is currently the common screening method for cervical cancer in the region. The NAC pilot hopes to have TruScreen
View
News, 20.10.2021
Clinical Trial Results Highlight Efficacy of TRU Technology
A new study, published in the European Journal of Obstetrics and Gynaecology and Reproductive Biology[1], concludes that TruScreen’s cervical cancer screening technology meets or exceeds the effectiveness of alternative cervical
View
News, 20.10.2021
TruScreen device – milestones achieved on growth strategy
TruScreen Group Limited is pleased to announce the achievement of key milestones relating to the ongoing development of the Company’s women’s health care technology and its overseas growth strategy.
View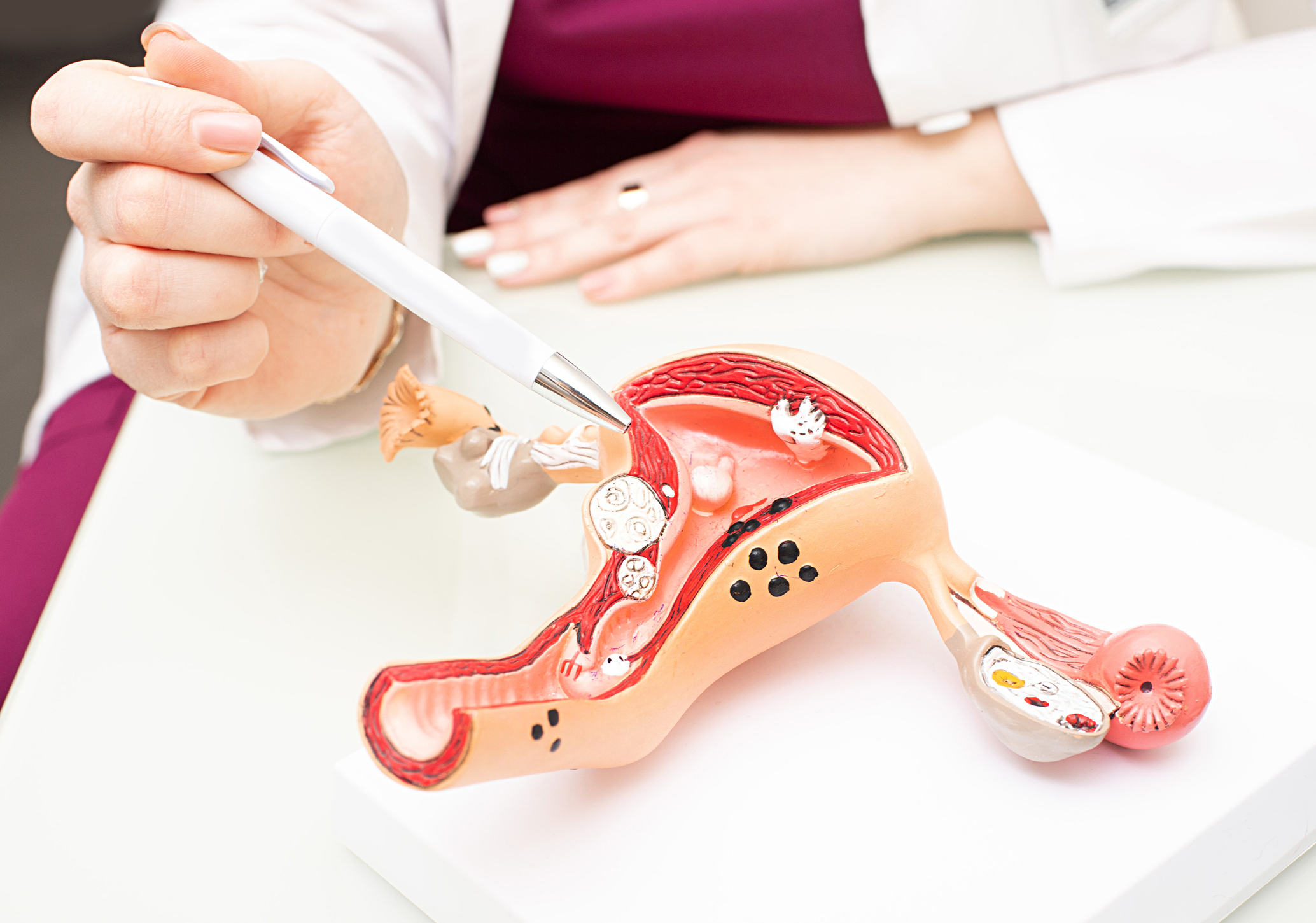
News, 9.2.2023
ASX Article
ASX Article | Wed 08 February 2023 | Tony Featherstone Move turns spotlight on medtech’s cervical-cancer screening device.
View
