Company Information
TruScreen Group Limited (NZX/ASX: TRU) is a New Zealand-based medical device company that has developed an AI-enabled device that can detect precancerous and cancerous cervical changes in real-time via optical and electrical measurements of cervical tissue. Unlike many cervical screening devices, that only have triage/adjunct functionality, the TruScreen device is registered as a primary screening tool.
TruScreen’s cervical screening technology effectively resolves many of the ongoing issues with cytology, including failed samples, poor patient follow-up, patient discomfort, and the need for supporting laboratory infrastructure.
The device is CE-marked, meaning it meets EU safety, health and environmental protection standards required for sale and use throughout Europe. It is also National Medical Products Administration approved for sale in China.
TruScreen is currently targeting product sales to a range of low and middle-income countries, including China, Mexico, Vietnam, Russia, and Saudi Arabia, where no large-scale cervical cancer screening programs and infrastructure are currently in place. By doing so, the we hope to help improve the health and wellbeing of women worldwide.
The TruScreen cervical cancer screening device offers an alternative approach to conventional cytology-based screening. It has comparable sensitivity to high-quality cytology in a gynaecology clinic, and resolves many of the ongoing issues with traditional methods.
TruScreen manufactures and owns all rights to the TruScreen Cervical Cancer Screening System. The system comprises a medical device and process designed to detect the presence in real time of pre-cancerous and cancerous tissue on the cervix.
Learn more about our technology
Recent News

News, 6.3.2021
International Women’s Day: Equal access to healthcare for all women
International Women’s Day is a global event marking the celebration of the social, economic, cultural and political achievements of women – while also making a call to action for accelerating
View
News, 27.11.2019
How TruScreen’s real-time cervical cancer screening works
TruScreen’s breakthrough cervical cancer screening device is a viable and attractive alternative to traditional cervical screening methods. The TruScreen device resolves many of the ongoing issues associated with Pap tests,
View
News, 7.11.2019
Five Facts You May Not Know About Cervical Cancer
Cervical cancer affects millions of women worldwide. It is a silent killer, it has a high prevalence outside of developed countries, and almost all cases of cervical cancer can be
View
News, 3.10.2019
Cervical Cancer Remains a Global Health Issue
Cervical cancer is the fourth most common cancer in women, with more than half a million women diagnosed with the disease in 2018. In low- and middle-income countries (LMIC’s) cervical
View
News, 9.1.2020
Social stigma restrains cervical cancer screening in China
Despite its rapidly growing economy, China remains a low- and middle-income nation. An example of this is its healthcare system. Although China has made impressive strides in improving healthcare access
View
News, 20.11.2020
TruScreen CEO interview with Proactive
TruScreen’s CEO Victoria Potarina, speaks to Andrew Scott from Proactive, ahead of the upcoming ASX dual listing and capital raise.
View
News, 10.12.2020
Zimbabwe’s dual public health crisis: cervical cancer and HIV
Zimbabwe may be known for its pristine wilderness, but it is also a country facing a major public health crisis in the form of high HIV and cervical cancer rates.
View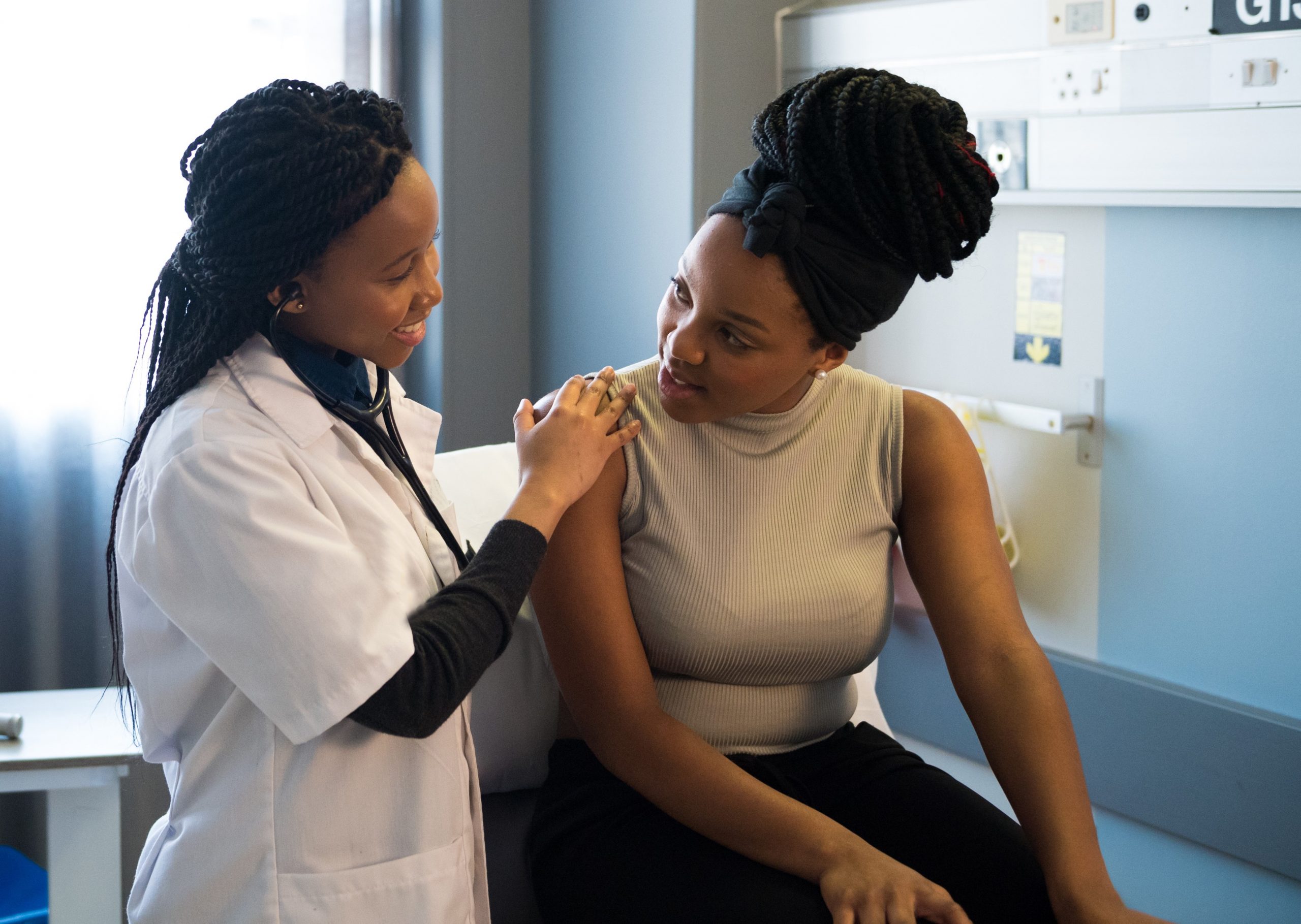
News, 22.10.2019
World Health Organisation sets global agenda to eliminate cervical cancer
Cervical cancer is one of the most preventable and treatable forms of cancer, yet over a quarter of a million women die from the disease each year. Ninety percent of
View
News, 15.5.2020
Video: TruScreen Clinical Validation and Application – Assoc. Prof. Michael J. Campion
Associate Professor Michael J. Campion, TruScreen Medical Advisory Committee member, sits down to discuss the science behind TruScreen’s Cervical Cancer Screening Device, its clinical validation, and its application in real-world
View
News, 12.6.2019
Cervical Cancer Technology Landscape – Unitaid & WHO Publication
As announced last month, TruScreen's cervical cancer screening device was recognised in a joint publication that Unitaid has released with the World Health Organisation and the Clinton Health Access Initiative.
View
News, 1.9.2021
TruScreen August ’21 Newsletter
Truscreen Group Limited (NZX/ASX: TRU) is pleased to provide a copy of its August 2021 Stakeholders Newsletter. This gives an update on TruScreen's performance and highlights key initiatives underway. Highlights
View
News, 26.10.2021
Wave of Health – the all-Russian Promotion
In September 2021, TruScreen’s Russian distributor, Intelmed Systems, participated in the 15th annual “Wave of Health” initiative. The annual “Wave of Health” campaign sees a ship with teams of doctors
View
News, 6.9.2021
Zimbabwe Pilot Program Underway
The National Aids Council pilot will compare TruScreen to VIAC. VIAC is currently the common screening method for cervical cancer in the region. The NAC pilot hopes to have TruScreen
View
News, 20.10.2021
TruScreen device – milestones achieved on growth strategy
TruScreen Group Limited is pleased to announce the achievement of key milestones relating to the ongoing development of the Company’s women’s health care technology and its overseas growth strategy.
View
News, 20.10.2021
Clinical Trial Results Highlight Efficacy of TRU Technology
A new study, published in the European Journal of Obstetrics and Gynaecology and Reproductive Biology[1], concludes that TruScreen’s cervical cancer screening technology meets or exceeds the effectiveness of alternative cervical
View
News, 9.2.2023
Zimbabwe second pilot project phase completed
The Zimbabwe National AIDS Council (NAC) wrapped up its current TruScreen pilot program in January. 10,000+ women were screened during the 6-month pilot. For many of these women this is
View
News, 18.1.2023
TruScreen visits Vietnam
TruScreen’s Commercial GM travelled to Vietnam in late 2022 as boarders reopen and pandemic pressures begin to ease.
View
Our People
TruScreen has a wealth of experience, highly skilled professionals across its Directors, Executive Team, Medical Advisory Board, and Research and Development Team.
Meet the teamOur History
The TruScreen technology’s development was driven by two leading medical academics from Sydney University, who sought to establish objective technology that could improve on the conventional Pap smear test. The first generation of the Truscreen device was commercialised and launched to the market in 2014.
Discover our history